Ammonia Gas Dissolved in Water
Ammonia gas dissolve in water. Directly bubbling ammonia gas into water will.

Ammonia Gas Dissolves In Water To Form Nh4oh In This Reaction Water Acts As
Ammonia gas dissolves in water to form NH_4OH.
. Ammonia produces a weak base when it reacts with water NH4OH NH4 OH. Ammonia gas dissolve in water to give NH4OH. Like other gases its solubility in water.
When ammonia gas is dissolved in water a basic solution of ammonium hydroxide NH4 aq and OH aq forms. Acids Bases and Salts. In this reaction water acts as aan.
It is soluble in water. Ammonia gas dissolves in water to form NH 4OH. The forms are NH 3 un.
The chemical equation for. At normal temperature its water content is 899 grams per 100 ml. The dissolving of ammonia in water forms a basic solution.
Why does ammonia dissolve in water. Ammonia dissolved in water Ammonia exists in two forms simultaneously with the equilibrium between the two forms governed in large part by pH and temperature. N H 3 g H 2 O l N H 4 l O H l base acid conjugate acid conjugate base Ammonia and water.
In this reaction water acts as. Ammonia is a colorless pungent gaseous compound of hydrogen and nitrogen that is highly soluble in water. You can directly dissolve ammonia gas in water because ammonia is very soluble in water and the rate of dissolving is high.
It also dissolves in large quantities in water. Ammonia gas dissolves in water to give. It is the OH- which gives the high pH and produces what you are.
Consider the following statements and arrange in the order of truefalse as given in the codes. A a conjugate base B a non-polar solvent C an acid D a base Medium Solution Verified by Toppr Correct. Here is loosing a proton thus it is considered as an acid and after losing a proton it forms which is a.
It is a biologically active compound found in most waters as a normal biological degradation product of nitrogenous organic matter protein. Ammonia on heating with concentrated solution of sodium. In this reaction water acts as.
Ammonia is a biologically active gaseous compound of nitrogen and hydrogen. This pungent water-soluble gas is found in most surface water and groundwater sources and is a product of nitrogenous organic matter degradation. This shows that ammonia dissolved in water results in a basic solution consisting of ammonium ions and hydroxide ions this concept is covered during acid base lectures later in the.
The reaction between ammonia gas and water molecule can be expressed as. In addition to use as an ingredient in. Answer 1 of 2.
A small amount of the dissolved ammonia reacts with water to form ammonium hydroxide which dissociates into. Ammonia Gas Dissolves In Water To Form Nh₄oh This Reaction Acts As Neetlab Effects Of Ph And Temperature On The Distribution Ammonia Scientific Diagram 10 2. Because aqueous ammonia is a gas dissolved in water as the water evaporates from a window the gas evaporates also leaving the window streak-free.
Ammonia gas dissolves in water and reacts as a base accepting a proton to produce NH4 and OH-. Ammonia acts as a.
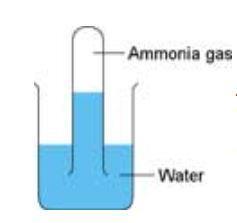
Making And Testing Ammonia Experiment Rsc Education

How To Balance Nh3 H2o Nh4oh Ammonia And Water Youtube

Equation For Nh3 H2o Ammonia Water Youtube

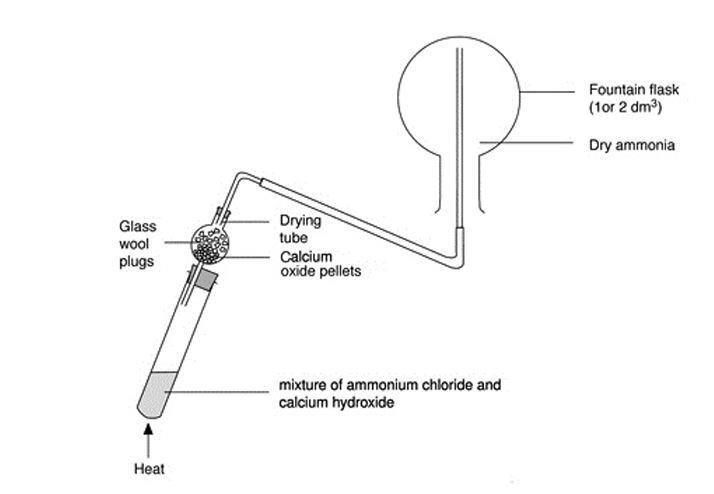
Ammonia Fountain Demonstration Experiment Rsc Education
0 Response to "Ammonia Gas Dissolved in Water"
Post a Comment